Address:Room 906, building 6, SIIC Center, No.195 Hong Kong East Road, Laoshan District, Qingdao city, Shandong Province, China
E-mail:info@deltachem.net

ØWhat is gas fumigation yellowing?
Gas fumigation yellowing of plastics refers to the phenomenon where plastic products turn yellow on the surface or internally due to oxidation reactions when exposed to environments containing nitrogen oxides (NOx). Nitrogen oxides (such as NO₂ and NO₃) react with amine stabilizers or organic components in plastics to form nitrosamine compounds. These reactions accelerate under high temperatures (e.g., above 60°C) or UV exposure, causing reduced light transmission and yellowing in the material.
ØKey Factors Contributing to Gas fumigation yellowing?
> Primary Factor: Nitrogen oxides, particularly NO₂.
Sources: Automotive exhaust, industrial emissions, gas stoves, boiler combustion, and other sources produce nitrogen oxides. Even seemingly clean indoor air may contain low concentrations of NOx sufficient to cause yellowing.
> Secondary factor: Improper antioxidant application
This is the crux of the issue. Many plastics (e.g., ABS, PA, PC) and coatings use hindered phenolic antioxidants during production to prevent oxidation-induced aging during processing and use.
Yellowing Process: Hindered phenolic antioxidants in the material gradually migrate to the surface. When these antioxidants on the surface react with NOx in the air through complex nitration reactions, they form yellow nitrobenzene derivatives—resulting in the visible “yellowing”.
ØHow to prevent and address NOx gas fumigation yellowing?
-- Using asymmetric hindered phenolic antioxidants or non-phenolic antioxidants resistant to gas-induced yellowing can effectively mitigate yellowing caused by NOx. OMNISTAB AO-80 and AN9142 provide effective solutions for this yellowing issue.
>> OMNISTAB AO-80
AO-80 is an asymmetric hindered phenolic antioxidant. Compared to traditional fully hindered phenolic antioxidants (where R1 and R2 are both tert-butyl groups), AO-80 exhibits relatively lower steric hindrance around its phenolic hydroxyl group. This facilitates nitro substitution at the meta position, effectively resolving NOx gas-induced yellowing. Conversely, traditional symmetric hindered phenolic antioxidants exhibit significant steric hindrance around the phenolic hydroxyl group, causing nitro substitution to occur at the para position. This frequently results in the formation of differently colored p-nitro compounds, explaining why phenolic yellowing is difficult to resolve.
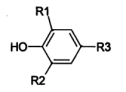
Hindered phenolic antioxidant structure
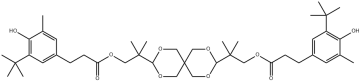
Structure of OMNISTAB AO-80
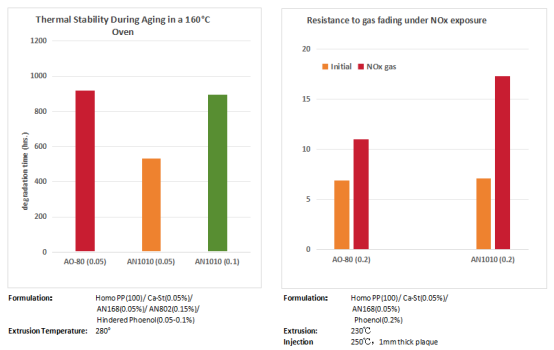
Test data from polyolefin demonstrates that OMNUSTAB AO-80 exhibits superior thermal stability compared to other hindered phenolic antioxidants. It resists fuming and yellowing under NOx exposure, providing sustained protection against thermo-oxidative aging during high-temperature processing and service. This maintains the material's initial color stability, significantly enhancing product quality and aesthetic appeal.
>> OMNISTAB AN9142
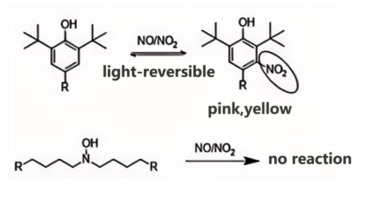
> AN9142 is a novel high-temperature-resistant, multifunctional antioxidant belonging to the hydroxylamine class. It effectively prevents material yellowing due to fumigation. In contrast, hindered phenolic antioxidants readily react with nitrogen oxides (NOx), thereby generating chromogenic byproducts.
> AN9142 exhibits excellent compatibility with polyolefin materials, low volatility, and migration resistance. It is particularly suitable for polypropylene fibers, automotive TPO, and systems demanding exceptional color stability and resistance to smoke-induced yellowing.
> Besides, during polyolefin extrusion processing, AN9142 delivers outstanding melt flow rate (MFR) stability.
> When AN9142 is used together with HALS, it exhibits excellent synergistic effects.
If you are interested in the above anti-yellowing antioxidants, welcome to further inquire and discuss!